Advanced Functional Suitability Testing
If the systems require initial closure penetration for final dosage form preparation, such as reconstitution, constitution, admixture, or dilution, the test conditions are designed to simulate such challenges. The tests required for individual packaging/delivery systems are shown in Table 1 of USP 382 and copied below:
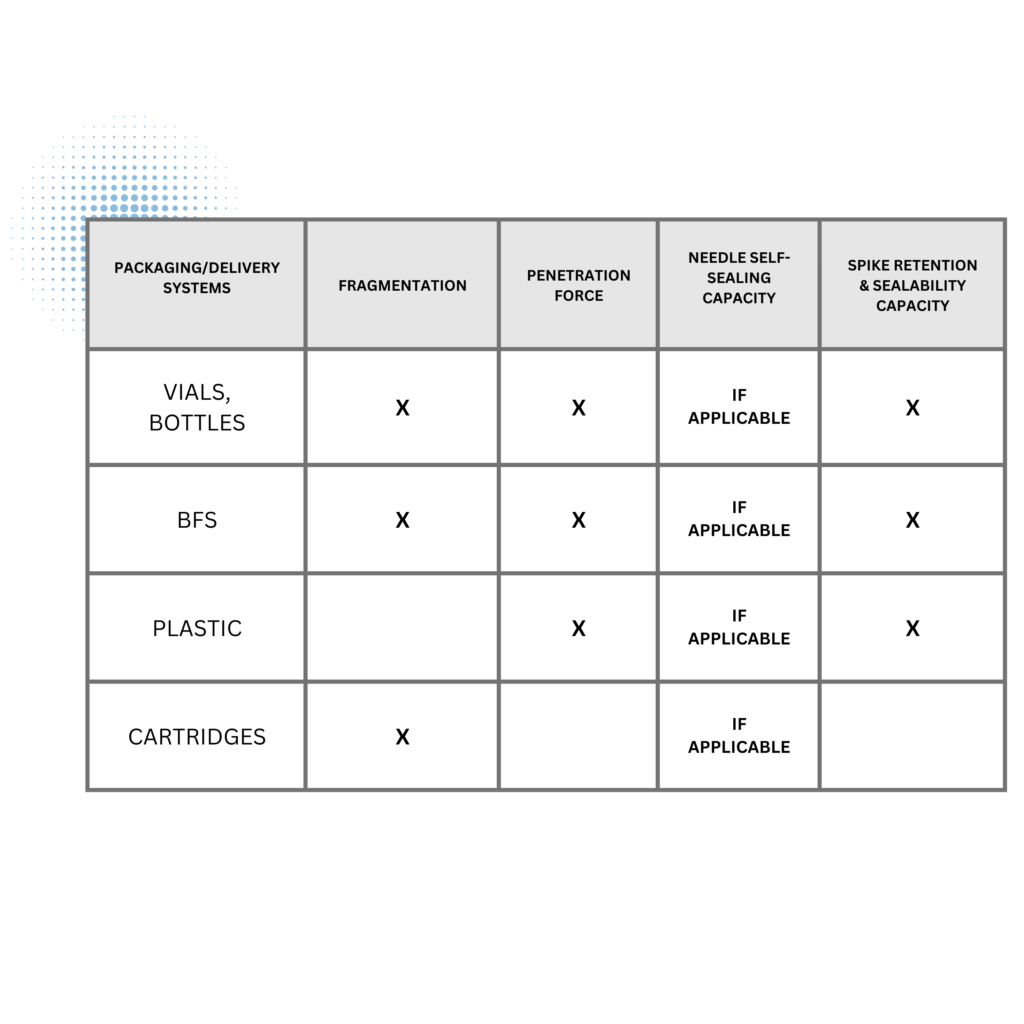
The functional suitability tests for needle and spike access apply to packaging and delivery systems that have closures permitting drug product access through a hypodermic needle, spike, or other closure penetration device. The four functional suitability tests are:
- Fragmentation
- Penetration Force
- Needle Self-Sealing Capacity
- Spike Retention & Sealability Capacity

Early-Phase Development
Comprehensive packages designed to navigate clinical & regulatory complexities of parenteral product-package and container-closure development, from concept to market readiness.
Container Closure
Integrity Testing
Quantitative, non-destructive, deterministic leak detection technologies for products stored in vials, syringes, cartridges, flexible containers, and bottles.
Particulate
Analysis
Multi-analytical methods to count, size, and characterize visible and subvisible particulate matter in pharmaceuticals, disposable containers, single-use systems, raw materials, and medical devices.
Medical Device
Analysis
Rigorous studies focused on enumerating particulate matter and identifying potential chemical compounds that could migrate from medical devices into the environment or directly impact patient health.
Extractables
& Leachables
Studies aimed at identifying potentially harmful compounds in manufacturing components and final containers to ensure the safety and efficacy of pharmaceutical products.

Covering every stage of the drug development journey with our state-of-the-art, FDA-inspected, cGMP-compliant facilities designed to minimize bottlenecks and maximize success.
