Container Closure Integrity Testing (CCIT)
At Gateway Analytical, we specialize in providing non-destructive deterministic Container Closure Integrity Testing (CCIT) methods designed to serve our pharmaceutical and biotechnology customers best. We offer laser-based gas headspace analysis, vacuum decay, and microcurrent high-voltage leak detection testing methods for products stored in vials or bottles, syringes, cartridges, flexible bags or pouches, dropper bottles, and drug-device combination products such as packages cased inside autoinjectors. Contact our experts today to learn how we help pharmaceutical and biotechnology companies develop and validate CCIT methods for their product-package configurations.
Testing and maintaining the integrity of nonporous packaging for sterile pharmaceuticals and biotech products is essential for upholding patient safety and quality standards, known as container-closure integrity assurance. The guide USP <1207> delves into evaluating the integrity of sterile product packaging, ensuring comprehensive regulatory compliance, quality.
Your Partners in Advanced CCIT Solutions
Ensuring the integrity of nonporous packages used for sterile pharmaceutical and biotechnology products is crucial to meeting patient safety and quality standards. Contact Gateway Analytical today to explore how we help companies develop and validate CCIT methods for their product-package configurations.

VeriPac Vacuum Decay Technology
VeriPac leak testers connect to a test chamber that is specially designed to contain the package to be tested. The package is placed inside the test chamber to which vacuum is applied. The single or dual vacuum transducer technology is used to monitor the test chamber for both the level of vacuum as well as the change in vacuum over a predetermined test time.
The changes in absolute and differential vacuum indicate the presence of leaks and defects within the package. The sensitivity of a test is a function of the package design, the package test fixture and critical test parameters of time and pressure. Test systems can be designed for manual or fully automated operation. This inspection method is suitable for laboratory offline testing and production applications for QA/QC statistical process control. The test cycle takes only a few seconds, is non-invasive and non-destructive to both product and package.
Vacuum decay, leveraging essential physical principles, serves as a method for assessing container integrity. Over years, this technique has been refined and enhanced through technological advancements.
Recognized for its efficacy and sensitivity among vacuum-based leak detection methods, vacuum decay offers dependable and precise quantitative outcomes, complete with clear pass/fail indicators.
The VeriPac inspection approach, rooted in vacuum decay technology, enables non-invasive testing of various packaging, including rigid, semi-rigid, and flexible types crafted from both non-porous and porous materials. PTI’s VeriPac instruments, employing this method, are adept at identifying leakages down to the micron level.

MicroCurrent HVLD Technology for Parenteral & Biologic Products
Gateway Analytical delivers traditional HVLD techniques, introducing an innovative approach to integrity testing for all parenteral and biological products, including low conductivity liquids like sterile water for injection. This novel method, dubbed Microcurrent HVLD, operates with approximately 50% less voltage, reducing the exposure of the product and environment to less than 5% of the voltage typically associated with conventional HVLD solutions.
Mentioned in USP <1207>, PTI’s HVLD technology stands as a highly sensitive method for detecting leaks in container closure integrity, applicable to a range of liquid-filled packaging such as pre-filled syringes, vials, cartridges, and ampoules.
The E-Scan procedure employs a series of electrode probes to examine a sealed non-conductive container, which may be made of glass, plastic, or poly laminate, requiring a minimum liquid fill of 30%. The presence of a pinhole, crack, or similar defect leads to a variation in resistance and current flow, signaling a compromise in the container’s integrity. This method also allows for the approximate location of the defect to be pinpointed.

Laser-Based Gas Headspace Analysis Technology for Cell and Gene Therapy Products (CGTs), Advanced Therapy Medicinal Products (ATMPs), and Lyophilized Products

LIGHTHOUSE’s FMS-Oxygen Headspace Analyzer:
Measures oxygen concentration in sealed parenteral containers. The benchtop headspace oxygen analyzer can be used throughout the entire product lifecycle, from development to QC laboratory applications to at-line, in-process control of headspace oxygen levels and production.
Applications Include:
- Leak detection
- Container closure integrity studies
- IPC monitoring of oxygen levels during the filling of oxygen-sensitive product
- Optimization and validation of purging systems on filling lines
- Oxygen degradation studies
- Stability trends, end-of-shelf life studies
- Packaging permeation studies
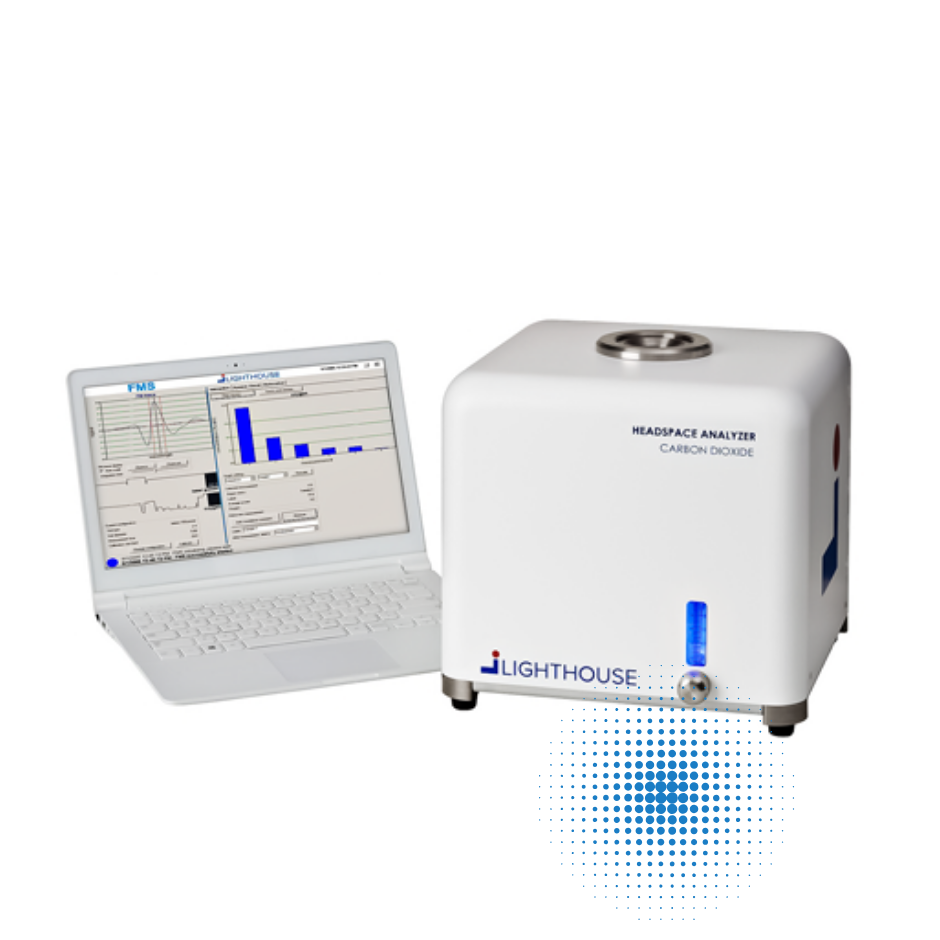
LIGHTHOUSE’s FMS-Carbon Dioxide (CO2) Headspace Analyzer:
Measures CO2 concentration in sealed parenteral containers for a variety of applications. The benchtop headspace carbon dioxide analyzer can be used throughout the entire product life- cycle, from development to QC laboratory applications to at-line, in-process control of headspace CO2 levels in production.
Applications include:
- Container closure integrity testing of frozen product stored on dry ice for transport
- General container closure integrity testing when the test method involves using carbon dioxide as a tracer gas
- IPC monitoring of carbon dioxide levels during the lling of product purged with carbon dioxide in the headspace
- Microbial growth detection in media vials
Defining Precision in CCIT
One of the most significant revisions to USP 1207 occurred in 2016, when a clear differentiation was made between probabilistic and deterministic leak test methods. A deterministic leak test method detects or measures a leakage event based on phenomena that follow a predictable chain of events.
The measure of leak detection is based on physicochemical technologies that are readily controlled and monitored, yielding objective quantitative data. A probabilistic leak test method is stochastic and relies on a series of sequential and simultaneous events, each associated with random outcomes described by probability distributions. The findings are associated with uncertainties requiring large sample sizes and rigorous test-condition controls to obtain meaningful results. Sample size and test condition rigor are inversely related to leak size.
The most significant effect of the delineation between probabilistic and deterministic CCIT methods comes from regulatory agency scrutiny. Regulatory agencies expect companies to select deterministic CCIT methods instead of probabilistic methods such as tracer liquid (dye ingress) or the microbial ingress challenge due to the availability of deterministic CCIT methods with greater quantification and the lowest detection limits.

About USP <1207>
USP <1207> defines essential terms such as container-closure integrity (CCI), which refers to a package’s ability to prevent product loss, block microorganism ingress, and limit the entry of detrimental gases or other substances. Container-closure integrity testing (CCIT) refers to any package leak test that detects the presence of a package breach or gap. A container-closure system (CCS) is the sum of CCS components and materials containing and protecting the article, including primary and secondary packaging components when required to provide additional protection.
USP <1207> provides detailed information on many topics about integrity assurance, including product quality risks posed by container leaks of concern, package integrity and seal quality tests for large and small-volume container-closure systems, leaks and leakage rate, closure type and mechanics, product-package quality requirements, and the maximum allowable leakage limit, as well as inherent package integrity and the package integrity profile.

Early-Phase Development
Container Closure
Integrity Testing
Particulate
Analysis
Medical Device
Analysis
Extractables
& Leachables

Covering every stage of the drug development journey with our state-of-the-art, FDA-inspected, cGMP-compliant facilities designed to minimize bottlenecks and maximize success.
